Abstract
Response inhibition one of the crucial cognitive functions in humans. Neuroimaging studies revealed the critical role of the right presupplementary motor area and the right anterior cingulate cortex in response inhibition. These data were obtained mostly on right-handers; however, lateralization of this function could be related to manual lateral asymmetry in humans. We compared brain areas involved in simple response inhibition tasks performance (rings and letters were used as visual stimuli) and differences in the activated brain structures between groups of participants with different types of motor asymmetry by fMRI. 29 participants (23 right-handers and 6 left-handers) performed Go/NoGo task and Stop-signal task. In Go/NoGo task the participants had to ignore specific non-verbal stimuli (red rings). In Stop-signal task the participants had to ignore specific verbal stimuli (red letters). Results showed increased activation of the right cerebellar hemisphere only for right-handers in comparison with left-handers in Stop-signal task. Within-group analysis in the left-handers group revealed activation of the fusiform gyrus and the BA19 during Go/NoGo task in comparison with Stop-signal task. Significant differences have been demonstrated between right-handers and left-handers in simple response inhibition tasks which involve verbal stimuli processing. The right cerebellum structures have been activated during verbal information processing in right-handers in Stop-signal-task.
Keywords: Response inhibitionhandednessfMRI
Introduction
Executive functions (cognitive control) provide human goal behavior. Cognitive control has been described as a complex process which includes various functions – updating, shifting, and inhibition (Miyake et al, 2000). Updating is a function that actualizes information from working memory essential for performing the current task. Shifting is a function that allows redirecting attention between successive tasks. Inhibition is a function that allows suppressing common reaction in favour of one of a few less frequently demanded reactions if it is a better match for the current task. Inhibition is a key function of cognitive control. However, brain mechanisms of inhibition are not clear yet. The cognitive control is a meta-function that coordinates and controls other mental processes. Therefore, this function is never present by itself. To study this function either Go/NoGo or Stop-signal paradigm are commonly used. In Go/NoGo task subjects need to either respond to frequent non-target stimuli or silently count them and ignore rare target stimuli. Stop-signal task is a modified version of this paradigm. Subjects need to ignore any stimulus if it is coupled with a stop signal (an additional visual or auditory stimuli).
Problem Statement
Brain basis of response inhibition tasks
Functional MRI studies showed that during cognitive inhibition of response various brain structures are active. These studies mainly provide evidence of the major role of the right hemisphere. Meanwhile, in some studies both hemispheres were activated in stop-signal tasks. Neuropsychological studies in patients with left-sided brain lesions showed the importance of the left inferior frontal gyrus (IFG) for cognitive inhibition (Swick et al, 2008). However, the data were obtained mostly in right-handers. This reveals a problem of lateral brain organization of cognitive inhibition. It should be taken into account that lateralization of this function could be related to functional lateral asymmetry in humans. Meta-analysis of Swick and co-authors demonstrated that the right anterior insula and the pre-SMA (presupplementary motor area) were active in the inhibition tasks (Swick et al, 2011). Pre-SMA is the most anterior portion of the supplementary motor area (Matsuzaka et al, 1998). In the Go/NoGo task the right insula (BA 13), the right middle frontal gyrus (BA 9), the right inferior parietal lobule/precuneus (BA 40, 19, 7), the superior frontal gyrus (medial BA 6, 8), the left middle and inferior frontal gyri (BA 9, 6, 44) and the left insula/putamen/claustrum showed the highest activation. In the Stop-signal task the left insula, subcortical structures (thalamus and putamen), the posterior cingulate cortex (BA23), the right insula, inferior and precentral gyri (BA 9), the superior frontal gyrus (medial BA 6), the right middle frontal gyrus (BA 9), and the right inferior parietal lobule (BA 40) were active. This reveals the cerebral asymmetry of cognitive control.
Sources of differences in inhibition tasks
Meta-analysis of Simmonds and colleagues showed that the differences in brain activation is coupled with the difference in task complexity (Simmonds et al, 2008). The authors argued that the more complex tasks are performed the more working memory resources are required. They also showed increased role of the pre-SMA in complex tasks. In more recent studies it has been stated that other mental processes than inhibition are involved in Go/NoGo, for instance attention and working memory (Criaud & Boulinguez, 2013). To prove this Criaud and Boulinguez have performed a meta-analysis specifically of the studies in which only complex Go/NoGo tasks were used (Criaud & Boulinguez, 2013). The complexity was measured according to the amount of Go and No/Go stimuli, the frequency of NoGo stimuli in the sequence, the occupacity of working memory (relationship between NoGo stimuli and demanded responses). This procedure has revealed the following brain structures to be involved in these tasks: the inferior parietal lobule, the middle frontal gyrus, the inferior frontal gyrus, the supramarginal gyrus, the middle frontal gyrus, the superior frontal gyrus, the inferior frontal gyrus, the superior temporal gyrus, the medial frontal gyrus. According to the study of Criaud and Boulinguez, in more complex tasks the right dorsolateral prefrontal cortex (DLPFC), the right inferior frontal gyrus and the pre-SMA were most active. The authors explained this as embeddedness of working memory in these tasks rather than inhibition. This hypothesis is strengthened by the intrinsic multifunctionality of those tasks typically classified as involving either shifting or inhibition or updating, which requires working memory, actually involve all these functions. The working memory is usually considered to be just one of the mental processes activated during the complex tasks. In the Conflict monitoring theory a conflict occurs when a subject has to choose one of a few possible responses. Upon giving the answer the evaluation and error monitoring take place. ERP studies associate this process with ERN (event related negativity) and Pe (posterror positivity) components. Source localization studies proved that both components are generated in the ACC and the pre-SMA (Brazdil et al, 2005; van Veen & Carter, 2002; Herrmann et al, 2004; Overbeek et al, 2005). This means that involvement of the structures mentioned above could be associated not only with working memory but with error monitoring as well: in complex tasks the number of errors could also increase. Executive functions, attention and error monitoring are interrelated processes as it was shown in several ERP studies. The ERN component correlates with efficiency of completing the tasks which involve these functions (Larson & Clayson, 2011).
Research Questions
In order to distinguish inhibition from other processes it seems more productive to use simpler tasks than it was done in the meta-analysis of Criaud and Boulinguez, 2013. The complexity of the task directly correlates with the amount of working memory involved as well as the reduced error monitoring. Therefore, there are fewer mistakes for less complicated tasks. This is why we used simple Go/NoGo and Stop-signal tasks with different sets of stimuli: color rings (non-verbal) and letters (verbal). The simplicity of the tasks was assured by the 50% frequency of No/Go stimuli in the sequence, characteristics of the stimuli set, monovariant association between the stimulus (red colored) and demanded response inhibition. It is supposed that Stop-signal tasks involve greater resources of working memory, meanwhile Go/NoGo tasks only require motor inhibition; involvement of working memory in these tasks is minimal.
Purpose of the Study
The primary goal of the study was to detect the functional asymmetry of the brain structures involved in response inhibition in participants with different patterns of motor asymmetry.
Research Methods
Participants
Twenty-nine mentally healthy participants with no history of neurological or mental disorders were recruited for this study (10 female, 19 male; mean age = 25.9±4.6). Among them there were 6 left-handers and 23 right-handers. Hand preference was determined according to Annett Hand Preference Questionnaire (Annett, 1970).
Procedure and materials
The main experiment included response inhibition in Go/NoGo paradigm and Stop-signal task. In the Go/NoGo (non-verbal) task a participant viewed as a background a monochromatic image of a butterfly in the centre of the screen with black ground color. The image of a butterfly strengthened perceptual complexity of the task. Then appeared a target stimulus which required a response (Go) or an inhibition of response (NoGo). These stimuli were color rings appearing on the left or on the right side from the butterfly. Color and place of the stimuli changed in quasi-random order. A participant was given the following instruction: “Press the left button of the computer mouse if a green ring appears on the left of the butterfly. Press the right button of the computer mouse if a green ring appears on the right from the butterfly. Meanwhile, if a red ring appears on any side of the butterfly, do not respond”. There were 50% of Go stimuli and 50% of NoGo stimuli in the sequence. In Stop-signal (verbal) task a participant was asked to press different buttons for green vowels and consonants that appeared on the screen. If a red letter appeared a participant was asked to ignore it. The participants were given the following instruction: “You will see green letters. If a vowel appears press the left button of the computer mouse. If a consonant appears press the right button of the mouse. If a red letter appears do not respond”. In the sequence there were 50% of stimuli which required an inhibition of response. Apart from these tasks the participants also completed other tasks (face perception and face recognition, a Stroop task and a hyperventilation task) which are not discussed in this article. The participants viewed the stimuli which appeared on the screen through a mirror secured inside the tomography. Before each task a participant heard the instruction through a loudspeaker. A block paradigm was used. For the first 15 sec of the session a participant was doing the task, then a 15 sec pause occurred. During the pause a participant viewed a fixation point on a black background. Each session lasted for 2.5 minutes.
MRI data acquisition
Whole-brain images were collected on 1.5 T General Electric Signa HDe scanner. Functional T2*-weighted echo planar imaging (EPI) data were acquired using axial slice orientation, repetition time (TR) = 3000 ms, echo time (TE) = 60 ms, flip angle = 90°, voxel size 3.75×3.75×5 mm3). High-resolution structural T1-weighted imaging data with 1 mm interval were acquired, TR = 11.1 ms, TE = 4.6 ms, flip angle = 13°, voxel size =1×1×1 mm3.
Data analysis
Data processing was comprised of several stages. First, images obtained during the brain scanning in the DICOM format (Digital Imaging and Communications in Medicine) were converted into NIfTI format (Neuroimaging Informatics Technology Initiative) using the Mipav Software. Then data was analyzed with fMRI Expert Analysis Tool (FEAT) and other tools using FMRIB Software Library v5.0 (FSL, www.fmrib.ox.ac.jk/fsl). The analysis included pre- and postprocessing. Preprocessing of each participant were analysed separately; artefacts and extracerebral elements were expelled. In postprocessing individual data were transformed to single standardized anatomic space MNI-152 (Monreal Neurological Institute template).
Preprocessing included reorientation to standard orientation, image correction (to exclude motion artefacts and magnetic field nonuniformity), 3D visual normalization, Gaussian kernel spatial smoothing (using 5 mm), and high-pass temporal filtering (cutoff: 100 s). Individual activation was obtained using a Z treshold 2.3 and a (corrected) cluster significance threshold of p=0.05. Both individual and group maps were drawn using the general linear model. Mean group activation for right-handers > left-handers contrast as well as left-handers > right-handers contrasts was modeled for Go/NoGo and Stop-signal tasks contrasts separately using a Z treshold 1.9 and a (corrected) cluster significance threshold of p=0.05 (between-group analysis). Mean group activation for Go/NoGo > Stop-signal contrast was modeled for righ- and left-handers separately using a Z treshold 1.7 and a (corrected) cluster significance threshold of p=0.05 (within-group analysis). The obtained coordinates of activation clusters are presented with the standardized anatomic space MNI-152. Coordinates of the brain structures are given according to the MNI Structural Atlas. Cortex structure coordinates were verified conformably to the Harvard-Oxford Cortical Structural Atlas. Coordinates of the cerebellum structures were verified conformably with the Cerebellar Atlas in MNI-152 space.
Findings
Right-handers > left-handers contrast
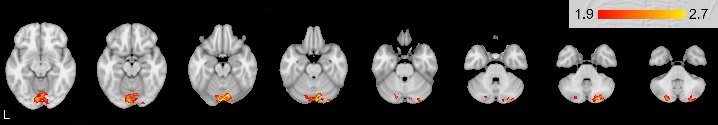
Mean group activation was revealed in the right cerebellum: posterior lobe VI, Crus I specifically in Stop-signal task (see Figure
Left-handers > right-handers contrast
Activation was not observed.
Go/NoGo > Stop-signal contrast in right-handers
Activation was not observed.
Go/NoGo > Stop-signal contrast in left-handers
Mean group activation was found in the right lateral occipital cortex (inferior division), the right occipital fusiform gyrus, Brodmann area 19.
Conclusion
In the current study we investigated the functional system of brain structures and the functional asymmetry of the brain structures involved in response inhibition in participants with different patterns of motor asymmetry.
Differences between right- and left-handers
The Go/NoGo activated areas are similar in right- and left-handers. Analysis between groups of right-handers and left-handers revealed differences only in Stop-signal task. The differences were observed in the right cerebellum activation (VI, Crus I). The importance of the cerebellum for programing, regulation and control was discovered by A. R. Luria and colleagues in 1964; it was even present in the name “pseudo-frontal syndrome” (Budisavljevic & Ramnani, 2012). More and more studies suggest that the cerebellum is involved in a variety of mental processes: attention, working memory, visuospatial functions, linguistic processing, word fluency, instrumental learning, executive functions (Molinari & Leggio, 2007). The Crus I and the Crus II are connected with the premotor and the prefrontal areas (Habas et al., 2009). Connections are mostly contralateral: 70-80 % of projections from the right hemisphere of cerebrum arrive at the left hemisphere of the cerebellum (Schmahmann & Pandya, 1997). Interconnnections enable cerebellum involvement in cognitive functions. The observed right cerebellum activation may be associated with verbal processing in the left cerebrum. This processing was strengthened by greater right cerebellum activation (VI, Crus I) in the group of right-handers. The present activation of the cerebellar VI, Crus I during verbal Stop-signal task is consistent with previous studies. Küper et al. showed that these areas are involved in working memory (Küper, 2016). Its activation was increased in response to growing N in N-back task. Ng and colleagues revealed increased activation of these structures of right cerebellum only in verbal working memory task, while in visual working memory tasks both cerebellar hemispheres were active (Ng et al, 2016). Our study has shown that features of verbal processing mechanism differ for right-handers. The observed differences between groups provide evidence that mechanism of simple non-verbal stimuli processing and inhibition of motor response to these stimuli in Go/NoGo task is common for people with different patterns of asymmetry. However, verbal stimuli processing in Stop-signal task is performed differently between groups of participants with different patterns of motor asymmetry. At the same time, any differences in activation of other structures between right- and left-handers were not found. These findings suggest that differences between right- and left-handers may be related to not response inhibition but stimuli type features due to interconnections the right cerebellum with the left cerebrum hemisphere. Thus, the structures of the right cerebral hemisphere are directly involved in control functions both in right-handers and left-handers.
Our study showed that simple tasks requiring response inhibition do not necessarily involve prefrontal brain structures. This corresponds with other studies (Kolodny et al, 2017).
Differences between Go/NoGo and Stop-signal task
Within-group analysis did not reveal any differences between two tasks in right-handers, which proves the common mechanism of solving this type of tasks. However, within-group analysis showed differences in left-handers. The increased activation in Go/NoGo task in comparison to Stop-signal task was observed in the right lateral occipital cortex (inferior division), the right occipital fusiform gyrus, Brodmann area 19 in response to non-verbal stimuli processing.
Acknowledgments
The research was supported by the Russian Science Foundation (project № 16-18-00066).
References
- Annett, M. (1970). A сlassification of hand preference by association analysis. British Journal of Psychology, 61(3), 303-321.
- Brazdil, M., Roman, R., Daniel, P., & Rektor, I. (2005). Intracerebral error-related negativity in a simple go/no-go task. Journal of Psychophysiology, 19, 244–255.
- Budisavljevic, S., & Ramnani, N. (2012). Cognitive deficits from a cerebellar tumour: a historical case report from Luria's Laboratory Cortex, 48(1), 26–35.
- Criaud, M., & Boulinguez, P. (2013). Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neuroscience and Biobehavioral Reviews, 37, 11–23.
- Herrmann, M. J., Römmler, J., Ehlis, A., Heidrich, A., & Fallgatter, A. J. (2004). Source localization (LORETA) of the error-related-negativity (ERN/Ne) and positivity (Pe). Cognitive Brain Research, 20, 294–299.
- Kolodny, T. , Mevorach, C. , & Shalev, L. (2017). Isolating response inhibition in the brain: Parietal versus frontal contribution. Cortex, 88, 173–185.
- Küper, M., Kaschani, P., Thürling, M., Stefanescu, M. R., Burciu, R. G., Göricke, S., Maderwald, S., Ladd, M. E., Hautze, H., & Timmann, D. (2016). Cerebellar fMRI activation increases with increasing working memory demands. The Cerebellum, 15(3), 322–335.
- Larson, M. J., & Clayson, P. E. (2011). The relationship between cognitive performance and electrophysiological indices of performance monitoring. Cognitive, Affective, & Behavioral Neuroscience, 11 (2), 159–171.
- Matsuzaka, Y., Aizawa, H., & Tanji, J. (1992). A motor area rostral to the supplementary motor area (presupplementary motor area) in the monkey: neuronal activity during a learned motor task. Journal of Neurophysiology, 68 (3), 653–662.
- Miyake, A., Friedman, N. P., Emerson, M. J., Witzki, A. H., Howerter, A., & Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41 (1) 49–100.
- Molinari, M., & Leggio, M. G. (2007). Cerebellar information processing and visuospatial functions. The Cerebellum, 6, 214–220.
- Ng, H. B., Kao, K. L., Chan, Y. C., Chew, E., Chuang, K. H., & Chen, S. H. (2016). Modality specificity in the cerebro-cerebellar neurocircuitry during working memory. Behavioural Brain Research, 305, 164–73.
- Overbeek, T. J. M., Nieuwenhuis, S., & Ridderinkhof, K. R. (2005). Dissociable components of error processing: On the functional significance of the Pe vis-à-vis the ERN/Ne. Journal of Psychophysiology, 19, 319–329.
- Schmahmann, J. D., & Pandya, D. N. (1997). The cerebrocerebellar system. In J.D. Schmahmann (Eds.), The Cerebellum and Cognition (pp. 31–60). San Diego: Academic Press.
- Simmonds, D. J., Pekar, J. J., & Mostofsky S. H. (2008). Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia, 46, 224–232.
- Swick, D., Ashley, V., & Turken, A. U. (2008). Left inferior frontal cortex is critical for response inhibition. BMC Neuroscience, 9, 102.
- Swick, D., Ashley, V., & Turken, A. U. (2011). Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. NeuroImage, 56, 1655–1665.
- van Veen, V., & Carter, C. S. (2002). The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiology & Behavior, 77, 477–482.
Copyright information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
About this article
Publication Date
13 December 2017
Article Doi
eBook ISBN
978-1-80296-032-7
Publisher
Future Academy
Volume
33
Print ISBN (optional)
-
Edition Number
1st Edition
Pages
1-481
Subjects
Cognitive theory, educational equipment, educational technology, computer-aided learning (CAL), psycholinguistics
Cite this article as:
Marakshina, J., Buldakova, N., Korotkov, A., Vartanov, A., Kozlovskiy, S., Shirenova, S., Kiselnikov, A., Popov, V., & Baev, A. (2017). Effect Of Handedness On Response Inhibition: Fmri Study. In S. B. Malykh, & E. V. Nikulchev (Eds.), Psychology and Education - ICPE 2017, vol 33. European Proceedings of Social and Behavioural Sciences (pp. 248-255). Future Academy. https://doi.org/10.15405/epsbs.2017.12.24

